The following was originally published in Johns Hopkins Medicine’s Newsroom.
“We need to see what’s happening inside the skull, including the relative locations of blood vessels and cells and how their organization changes during injury and over time,” says Warren Grayson, Ph.D., professor of biomedical engineering and director of the Laboratory for Craniofacial and Orthopaedic Tissue Engineering at the Johns Hopkins University School of Medicine. His lab focuses on developing biomaterials and transplanting stem cells into the skull to re-create missing bone tissue. (That technology is available for licensing through Johns Hopkins Technology Ventures.)
Other scientists have provided maps of small portions of blood vessels and stem cells in the mouse skull. “However, a larger picture of the skull gives us a better understanding of the entire vasculature and distribution of different stem cell types,” says Alexandra Rindone, graduate student at The Johns Hopkins University and School of Medicine and first author of the paper.
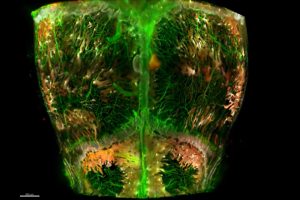
The new map, published Oct. 28 in Nature Communications, is a 3D view of the top of a mouse skull — its cranial bone, or calvaria — which is made up of four connected skull bones.
To create the map, which includes hundreds of thousands of cells, the Johns Hopkins researchers used four key techniques to pinpoint vessels and cells.
First, they used immunofluorescence to tag molecules on the surface of a variety of blood vessels and stem cells with a fluorescent, or glowing, chemical.
Then, the scientists use a chemical compound that helps light penetrate the skull without scattering — a method called optical tissue clearing. “It makes the skull appear like glass,” says Rindone.
To take the 3D image, the scientists used a lightsheet microscope, a device that takes images of large sections of tissue at high resolution and rapid speed, but minimizes photobleaching. “This tool helps us avoid deterioration of the fluorescent dye when tissues are exposed to light sources for a long time,” says Rindone.
Finally, they used computer software to identify and segment the skull’s 3D cellular structures and re-create the structures’ spatial coordinates and volumes. “This shows us the prevalence of stem and bone cells and their orientation in the skull,” says Rindone.
The map revealed previously unknown niches in the skull where stem cells reside, particularly near structures called the transcortical canals, which are small channels that penetrate the skull bone and connect the outer linings of the skull to the cavities in the center that contain bone marrow.
The Johns Hopkins scientists are working to adapt the 3D map-making method to image the human skull, which is challenging because of the human skull’s large size and how light passes through it. Yet, the method could be used to make 3D maps of cell types within bone and other human tissues.
The research was supported by the National Science Foundation, the National Institutes of Health’s National Institute for Dental and Craniofacial Research (F31DE029109, R01DE027957), the ARCS Foundation Metropolitan Washington Chapter and the National Institutes of Health’s Shared Instrumentation Grant (1S10OD020152-01A1).
Other scientists who contributed to the work include Xiaonan Liu from Nanfang Hospital, Southern Medical University in China; Stephanie Farhat and Daniel Coutu at the University of Ottawa; and Alexander Perdomo-Pantoja, Timothy Witham and Mei Wan from Johns Hopkins.