In a promising leap forward for neurofibromatosis type 1 (NF1) research, scientists at Johns Hopkins have developed a gene therapy approach that could fundamentally alter the course of the disease. The initiative centers on replacing the dysfunctional or absent neurofibromin protein in NF1 tumor cells using a bioengineered adeno-associated virus (AAV) vector.
NF1 affects roughly 1 in 3,000 people worldwide, causing tumors to grow on nerves throughout the body. While some tumors remain benign, others can become malignant and life-threatening. Current treatment options are limited, leaving patients and families with few choices.
Renyuan Bai, MBBS, PhD , Associate Professor of Neurological Surgery, and his team at Johns Hopkins are working to change that through an innovative gene therapy approach that could transform NF1 care.
, Associate Professor of Neurological Surgery, and his team at Johns Hopkins are working to change that through an innovative gene therapy approach that could transform NF1 care.
NF1 manifests in three primary forms:
- Cutaneous neurofibromas: Noncancerous tumors of the skin that cause pain, disfigurement, and therefore profoundly affect quality of life.
- Plexiform neurofibromas: Tumors that grow on larger nerves inside the body and can cause major neurologic dysfunction or be malignant.
- Malignant peripheral nerve sheath tumors (MPNST): Aggressive cancers with high mortality rates.
“Essentially, the only targeted therapy across these forms is MEK inhibitors for plexiform tumors,” Bai explains. “It’s a major unmet need.
The condition NF1 is caused by genetic variants in the NF1 gene, resulting in the loss of function of neurofibromin, a protein that normally suppresses the RAS signaling pathway. This is a critical regulator of cell growth. Without neurofibromin, RAS becomes hyperactive, driving uncontrolled cell growth and tumor formation.
“The biologic question was: is it possible to replace neurofibromin? And if you do, does it foundationally change the behavior of the cell and therefore the behavior of the disease?” said Jaishri Blakeley, MD, director of the Johns Hopkins Comprehensive Neurofibromatosis Center and Executive Director of the Neurofibromatosis Therapeutic Acceleration Program (NTAP), the predominant funder of the NF1 gene therapy work in the Staedtke/Bai laboratory. “We now know that it is possible.”
“Our idea is simple in theory: put neurofibromin back into the cell,” Bai says. “But the reality is much harder because neurofibromin is huge; it doesn’t fit into the viral vectors we typically use.”
To overcome this, Bai’s team focused on the protein’s central enzymatic domain, called GRD, which suppresses RAS activity. By engineering a modified GRD domain that attaches to the cell membrane where RAS resides, they created a smaller, functional payload suitable for AAV delivery.
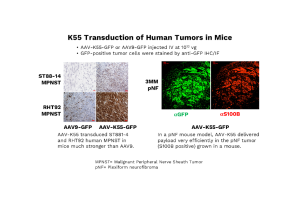
Delivering the therapy effectively posed another challenge: AAV vectors do not naturally target neurofibroma tumors. To address this issue, the team employed directed evolution—a process that uses a mutational library of AAV variants, which were injected into mice bearing human NF1 tumors. Variants that successfully localized to the tumors were then collected for further analysis. Through DNA shuffling and two different iterative selections, they identified a promising candidate: K55, a vector with strong affinity for human neurofibroma tissue.
“When we combined K55 with our GRD payload, we saw tumor suppression in mice,” Bai says. “That was a big moment for us.”
Toxicity studies in mice are now underway to reduce potential immune reaction to the vector and determine safe dosing limits. The next step is raising funds for non-human primate studies to evaluate safety. This is an essential precursor to an investigational new drug (IND) application and, ultimately, clinical trials in NF1 patients.
“NF1 is unique,” Bai said. “It’s almost a monogenic disease. If you can replace this gene, you can fix most of the problems. MPSNT might be more complicated, and we will address it by developing this therapy further.”
NTAP’s role has been pivotal. Known for its rapid funding cycles and collaborative ethos, NTAP helped bring forward the first FDA-approved drug for plexiform neurofibromas and is now supporting efforts seeking approval for cutaneous neurofibromas.
“NTAP is entirely focused on therapeutics,” Blakeley said. “Our goal is to scout talent, develop ideas, and accelerate them toward meaningful management and ultimately a cure.”
While the initial clinical application was aimed at cutaneous tumors, the focus has shifted to MPNST and glioblastoma, due to the devastating impact of these cancers and the preclinical activity observed.
The research team is now preparing for non-human primate studies, which require significantly larger vector volumes and commercial-grade production.
“We need to figure out the delivery and dose for efficacy in adults and need partners to do that,” Blakeley said. “This work could impact not just cancers in people with NF1, but also common sporadic cancers that often have NF1 alterations, including glioblastoma, breast cancer, melanoma, sarcoma, and resistant lung cancer.”
For NF1 tumors, the team is exploring complementary approaches, including oncolytic HSV therapy for localized tumor elimination, but gene therapy remains the centerpiece.
“This gene therapy is the way to go,” Bai concludes. “It’s not just science, it’s hope.”
